경피 패치제는 지속 점주에 필적할 만한 안정적인 약물 농도를 유지를 유지할 수 있게 도와주는 약물 전달 제형이다. Transdermal drug delivery enables the continuous systemic application of strong opioids through the intact skin, producing constant serum concentrations comparable with those achieved by continuous infusion.
1. Fentanyl 패치제의 Pharmacokinetic(25 mcg/hr)
*해당 study는 Durogesic SMAT (reference) vs Fentalgon® (test)의 생체동등성 비교 시험이지만 포스팅의 주제에 맞게 필요 부분만 수록하였다.
The aim of this study was to assess the relative bioavailability of fentanyl between two different fentanyl transdermal therapeutic systems, a new bilayer matrix type fentanyl transdermal patch (Fentalgon®, HELM AG, Germany) and a monolayer matrix-type patch (Durogesic® SMAT, Janssen-Cilag GmbH, Germany),with a releasing rate of 25 µg/h, after a single dose administration in healthy male subjects.
Methods
open label, single centre, randomized, single dose, two period crossover clinical trial
36명의 건강한 남성 지원자
| The mean age | 32.5 ± 6.3years |
| The mean body mass index | 24.0 ± 2.47 kg/m² |
Results
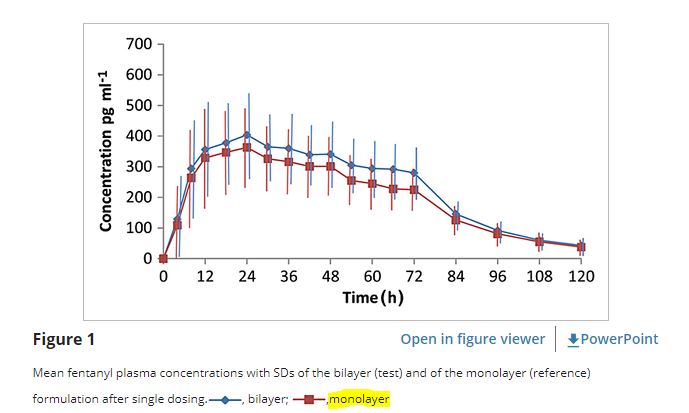
*300 pg/mL=0.3 ng/mL
2. Fentanyl 패치제의 Pharmacokinetic(100 mcg/hr)
https://gimi-drug.tistory.com/372
듀로제식 디트랜스(Durogesic D-trans patch): 잘라서 사용 가능? 절단 가능?
과거(약 2008년) 듀로제식(not 디트랜스) 패치가 리콜 대상이 되면서 국내에 있는 듀로제식 디트랜스 패치 역시 해당 제품이 아닌가에 대한 우려가 있었지만 다행히 각 제품의 패치 특징이 달랐다.
gimi-drug.tistory.com
A total of 30 healthy, male subjects were enrolled into the study. Their median age was 36 and ranged between 22 and 45 years; body weight (mean ± standard deviation) was 80.1 ± 10 kg. Overall, 28 subjects completed the study according to the study protocol. Due to high-intra-subject differences, two subjects were classified as outliers, and were excluded from the per protocol analysis; per protocol set consisted of 26 subjects.

* 1 mcg/L = 1 ng/mL

Fig. 3. Comparison of fentanyl delivery to healthy subjects (per protocol population). Comparison of fentanyl delivered from the transdermal patches to the healthy subjects over 72 h (study periods 1–4). The values were calculated from the initial patch drug load nominal values (11 mg for Matrifen® and 16.8 mg for Durogesic® DTrans® (marketed in Germany as Durogesic® SMAT)) and the measured residual fentanyl content in the used transdermal patches. The box–whiskers plots represent the amount of fentanyl in the transdermal patches. The top and bottom line of the box represent the 75th and 25th percentile, respectively; the line inside the box is the median of the data. The ends of the whiskers correspond to the minimum and maximum values. (A) The overall dose of fentanyl delivered per subject for Matrifen® was mean ± SD 9.05 ± 1.04 mg, median 9.32 mg and for Durogesic® DTrans® mean ± SD 8.79 ± 2.15 mg, median 8.90 mg. (B) The amount of residual fentanyl in used transdermal patches for Matrifen® was: mean ± SD 1.95 ± 1.04 mg, median 1.68 mg and for Durogesic® DTrans® mean ± SD 8.01 ± 2.15 mg, median 7.90 mg. (C) The percentage of the drug delivered from the initial patch for Matrifen® was: mean ± SD 82.3 ± 9.43%, median 84.7% and for Durogesic® DTrans® mean ± SD 52.3 ± 12.8%, median 53.0%.
즉,약 9000 mcg의 fentanyl이 72시간동안 방출됨을 의미, 약 125 mcg/hr

3. Fentanyl 패치제의 단회 부착 vs 다회 부착


4. Fentanyl 주사제의 continuous infusion Pharmacokinetic
We therefore investigated the population pharmacokinetics of fentanyl in a large cohort of critically ill patients who received fentanyl via infusions or multiple bolus doses. Our objective was to characterize fentanyl population pharmacokinetics in an ICU cohort and identify patient characteristics associated with altered fentanyl concentrations.
Methods
We conducted this prospective cohort study concurrently with the Bringing to Light the Risk Factors and Incidence of Neuropsychological Dysfunction in ICU Survivors (BRAIN-ICU) Study (11), which investigated risk factors for long-term cognitive impairment after critical illness. In the current study, we included all BRAIN-ICU subjects who received fentanyl at Vanderbilt University Medical Center during the first five days of their ICU stay.

a Categorical variables are reported at n (%), and all other variables are reported as mean ± standard deviation (range).
b Sepsis was identified using international consensus criteria on a daily basis during the patient’s hospitalization for up to 30 days.
c Because these variables are measured repeatedly within the same patient and are therefore not independent within a patient, summary statistics that are calculated based on individual measurements do not appropriately reflect population parameters. The median value was therefore calculated for each patient, and then summary statistics for the medians were calculated across all patients.
d The summary statistics were calculated based on individual.
e Two patients received phenytoin, one received itraconazole, and one received ritonavir.
이 관찰 연구에서, 펜타닐과 다른 모든 약물은 중환자실 팀의 재량에 따라 투여되었다.
48시간 동안 지속 점적 주입(continuous infusion) or 24시간 동안 2번 이상의 일시 주입(bolus)가 투입된 환자를 대상으로 최대 5일동안 매일 혈액을 채취하였다.
Results
<투여 방법의 특징>
| The mean duration of fentanyl infusion | 58 hours |
| The mean infusion rates | 131 mcg/hr |
| The median infusion rates | 100 mcg/hr |
| The median number of boluses per patient in the study period | 2 bolus |

5. 한계점
1) 환자군이 다르다. ex 나이, 기저력, 기저 신기능/간기능
2) head to head trial이 아니다.
6. 종합
Fentanyl IV continuous infusion하게 투여하여 serum fentanyl concentration을 평가한 시험은 드물었다. 하지만 여러 문헌을 종합하여 볼 때, 듀로제식 패치 옆에 기입된 숫자(100 mcg/hr, 50 mcg/hr 등)은 피부로 전달(방출)되는 fentanyl의 속도라고 생각할 수 있고 각각의 제형에 따라 100 mcg/hr로 투여 시 펜타닐의 농도는 각각의 그림에서 확인해 볼 수 있다. (거의 비슷한 혹은 IV 시 더 높은 extent라고 생각할 수 있겠다.)
뿐만 아니라 패치제의 경우에도 다회 부착 시 축적(accumulation)되는 것으로 보인다.
Fentanyl moves in the direction of the lower concentration at a rate determined by the matrix and the diffusion of fentanyl through the skin layers.
While the actual rate of fentanyl delivery to the skin varies over the 72-hour application period, each system is labeled with a nominal flux which represents the average amount of drug delivered to the systemic circulation per hour across average skin.
패치제 장점: Skin does not appear to metabolize fentanyl delivered transdermally. This was determined in a human keratinocyte cell assay and in clinical studies in which 92% of the dose delivered from the system was accounted for as unchanged fentanyl that appeared in the systemic circulation. ; 초회통과 효과를 덜 받는다.
👀 특정 환자군에서 fentanyl 패치제
| Hepatic Impairment 간부전 |
Renal Impairment 신부전 |
| Information on the effect of renal impairment on the pharmacokinetics of Duragesic is limited. | |
| 수술 환자를 대상으로 듀로제식 패치 50 mcg/hour을 72시간 부착 Compared to the controlled patients (n=8), Cmax and AUC in the patients with cirrhosis (n=9) increased 35% and 73%, respectively. |
IV fentanyl 25 mcg/hr Patients (n=8) undergoing kidney transplantation An inverse relationship between blood urea nitrogen(BUN) level and fentanyl clearance was found. > BUN과 fentanyl clearance가 역의 상관관계를 보였음 |
'🤹♂️ 카테고리별 약물 > 진통·진정' 카테고리의 다른 글
| 소아 환자의 진정/진통: pediatric analgo-sedation (0) | 2022.05.10 |
|---|---|
| 2021 성인중환자실에서 통증, 진정, 섬망, 부동화 및 수면장애 예방의 임상 진료지침 (0) | 2021.10.11 |
| Dexmedetomidine vs Propofol: 약제별 특성 비교(comparison) (0) | 2021.09.01 |
| 듀로제식 디트랜스(Durogesic D-trans patch): 잘라서 사용 가능? 절단 가능? (0) | 2021.08.31 |
| Opioid 특이 부작용: bile duct dilation, 담도 경련, 담낭 기능 부전, 급성 췌장염 (0) | 2021.07.24 |


댓글